
Entropy – 2nd Law
Definition of thermodynamic efficiency:


![]()
or

Consider a Carnot cycle: (page 157 5th Ed.)

For the isothermal steps:
A)

B)

From the adiabatic steps:
![]()
Since ![]() and
and


Integrating a® b or c® d:

Then:

From
A) & B):
Substitution for Neqn:


Clauusius’ theorem – any process can be broken into equivalent isothermal & adiabatic steps!
Therefore as before we can sum over the cycle:

or for many tiny cycles to sum up:

Over less than a whole cycle the integral may not be zero;
Then:

or

![]()
Combined 1st & 2nd Law
![]()
![]()

Substitute for dQ
![]()
or

or
![]()
Ideal Gasses & 2nd Law
1) Constant volume process:
![]()
![]()


![]()
![]()
![]()
.![]()
![]()


![]()



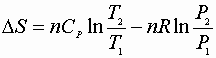

P, V: (P1V1® P2V2)


Since D S is a state function these can be used for any path!
Adiabatic reversible processes

Isentropic process!